Post-pasteurisation contamination is a troublesome obstacle for thedairy industry. The following fact sheet is compiled to provide a detailed picture of the problem in question and the solutions adopted by Piramide Environment, a company expert in the control and prevention of biofilm contamination.
Raw milk, although produced under ideal circumstances, has a different bacterial ecology that reflects the lifestyle of the animal and the environment in which it is produced. Raw milk contains a wide range of gram-positive and gram-negative bacteria, pathogens, spoilage bacteria and organisms that are commensal with the animal or cause disease to the animal. Fortunately, pasteurisation reduces the levels of many of these organisms by up to 6 orders of magnitude (Villamiel and de Jong, 2000).
Bacterial recontamination of pasteurised milk is a potential hazard, particularly if the milk is extensively handled and exposed to the environment after pasteurisation (e.g. cooling, bottling and cheesemaking; Koehler and Tonney, 1911). This recontamination, commonly referred to as post-pasteurisation contamination (PPC), often leads to sensory deterioration and reduced shelf life of the milk or finished product (Thomas and Druce, 1969; Hayes et al., 2002; Martin et al., 2018). It is estimated that post-pasteurisation contaminations are responsible for the loss of approximately 15% of dairy products in the industrialised world (Gustavsson et al., 2011).
The defects most associated with these contaminations are:
- Acidity, astringency
- Patchy taste
- Bitterness
- Coagulation
- Animal hints
- Fruity hints
- Loss of freshness
- Deterioration of fat (rancidity)
- Abnormal colouring/pigmentation
The causes of PPC can be attributed to two factors
- Contamination by micro-organisms less sensitive to heat treatment that are able to survive even when subjected to high temperatures
- Contamination by psychotolerant bacteria from poor equipment hygiene, biofilms, aerosols and/or incorrect handling by personnel (Sunga et al., 1970; Schröder, 1984; Eneroth et al., 2000b).
Heat-resistant bacteria

Heat treatments (pasteurisation and sterilisation) are the most widely used systems in thedairy and foodindustry in general to reduce/break down the microbial load.
However, not all micro-organisms are equally sensitive to such treatments, but there are micro-organisms that can survive even when subjected to high temperatures.
Some heat-resistant or thermoduric bacteria (e.g. Micrococcus) are able to survive pasteurisation conditions (e.g. 72°C/15s) in vegetative form (Gleeson et al., 2013). In addition, sporigenic bacteria can survive pasteurisation in the form of spores and several aerobic sporigenes have been identified that can grow under refrigerated conditions in pasteurised milk (Ivy et al., 2012).
Microorganisms such as Micrococcaceae, Streptococci, Lactobacilli, Bacillaceae and sometimes gram-negatives belong to this group.
In addition to being able to survive in milk, these micro-organisms can persist on the surface of implants forming a biofilm and represent an additional risk of post-pasteurisation contamination (Arizcun et al., 1998; Roberts and Wiedmann, 2003; Wong, 1998).
For example, Streptococcus thermophilus can survive the heat treatment required to pasteurise milk, and can subsequently colonise stainless steel surfaces downstream of the pasteuriser, forming a biofilm. It has also been shown that S. thermophilus cells, after colonising surfaces and producing biofilms, are more resistant to disinfectants and heat than their planktonic cell state (Flint et al., 1999, Flint et al., 2002).
These microorganisms can grow and survive even at refrigeration temperatures. Usually, gram-negatives introduced by a PPC cause rapid deterioration, reaching levels above 20,000 cfu/mL within a few days, before the subsequent growth of psychotolerant sporigenes occurs. In contrast, in the absence of PPC by gram-negative microorganisms, the psicrotolerant aerobic sporigenes present in raw milk typically reach levels capable of generating spoilage after 14 days at 6°C (Ranieri and Boor, 2009). We might expect much of the PPC to be caused by these aerobic sporigenic bacteria that originate in raw milk and survive pasteurisation, yet almost 50% of pasteurised milk shows signs of contamination by thermolabile but psychotolerant gram-negative bacteria that originate from the processing plant and are able to recontaminate the milk after pasteurisation.
Psychrophilic microorganisms and their impact in PPCs

Classical pasteurisation parameters (a minimum of 72°C for 15 s) provide a significant reduction in psicrotolerant gram-negative bacteria (Champagne et al., 1994), and at least a 6-log reduction in certain Pseudomonas species (Villamiel and de Jong, 2000). Thus, the presence of Pseudomonas and other gram-negative bacteria in pasteurised milk is typically an indication that a contamination event has occurred after processing.
Pasteurisation failures and the presence of high levels of gram-negative bacteria (resulting in the survival of some bacterial cells upon pasteurisation) in raw milk may also be responsible for the presence of gram-negative bacteria in finished products.
There are four primary groups of psychotolerant bacteria important in the PPC of milk:
- Pseudomonas: is by far the organism most reported to be responsible in milk PPC. Several factors contribute to the success of Pseudomonas as a PPC agent, the first being its ability to grow rapidly at low temperatures (Ternström et al., 1993). Furthermore, Pseudomonas is known to be particularly adept at competing with other deteriorating microorganisms, partly due to the ability of many strains to produce antibacterial and antifungal agents and siderophores (Gram et al., 2002), which are excreted in the growth medium where they bind and solubilise iron.
- Pseudomonas fluorescens has been described as the predominant (Law, 1979; Dogan and Boor, 2003; Brown and Luke, 2010) and most important species of Pseudomonas found in pasteurised milk. Other species that have been detected in milk include Pseudomonas fragi, Pseudomonas lundensis and Pseudomonas putida (Ternström et al., 1993; Eneroth et al., 2000b; Brown and Luke, 2010).
- Click here to read the in-depth article on Pseudomonas
- Coliforms: are a group of facultative, gram-negative, non-spore-forming aerobic and anaerobic rods that are able to ferment lactose to produce gas and acid within 48 hours at 32-35°C (Davidson et al., 2004). Most coliforms belong to the Enterobacteriaceae family.
- Non-coliform gram-negative bacteria: Among the non-coliform gram-negative bacteria associated with post-pasteurisation milk spoilage are mainly Aeromonas, Flavobacterium, Alcaligenes, Acinetobacter (Sørhaug and Stepaniak, 1997). Like Pseudomonas and coliforms, this group of bacteria has been shown to include several species and strains that can grow at low temperatures and produce a variety of thermostable enzymes that lead to organoleptic defects and abnormal milk coagulations (Michener and Elliott, 1964).
- Gram-positive sporigenic bacteria: gram-positive bacteria, including aerobic gram-positive sporigenes, are also capable of contaminating milk after pasteurisation; however, several gram-positive bacteria also survive pasteurisation, either in vegetative form (e.g. Micrococcus; Gleeson et al, 2013) and spores (e.g. Paenibacillus; Postollec et al., 2012), making it more complicated to determine whether these types of organisms, when found in finished products, originate from raw milk or PPC.
PPC of milk by psychotolerant spoilage bacteria plays a significant role in limiting the quality and shelf life of pasteurised milk. In fact, it has long been recommended that special attention be paid to the operation of the pasteuriser in view of the fact that it can become a casual source of infection if not handled properly’ (Smith, 1920).
The role of the pasteuriser in the onset of PPC

In the dairy industry, plate heat exchangers are commonly used to pasteurise milk and other products (Shah et al., 1988). However, when milk flows through the plates, the milk proteins are denatured due to the heating of the milk and stick to the surface of the equipment (Rosmaninho and Melo, 2006). The composition of the deposit on the plates is phosphorus, calcium and proteins. The composition of this deposit varies according to the different sections of the exchanger: in areas where the temperature is constantly maintained > 70°C, the protein deposit is the main one, about 133 mg/plate.
One of the reasons for the poor effectiveness of pasteurisation is the presence of biofilm and biofouling in the equipment. Milk fouling and the presence of biofilm are costly and persistent problems for the dairy industry. The fouling layer accelerates the adhesion of bacteria and promotes the development of biofilms (Sadeghinezhad et al. 2015).
The presence of deposits on heat exchanger plates is therefore undesirable for both economic and technical reasons. Production can be subject to problems related to both the spread of bacteria in the product, leading to microbiological problems, and the failure to restore the heat transfer characteristics of the plates (Sandu and Singh, 1991). A layer 250 microns thick can result in a 50% reduction in heat transfer (Goodman 1987). Finally, metal surfaces are corroded (Bryers, 1987; Gupta and Anand, 2017) due to the metabolic activity of the microorganisms present in the biofilm. All this also implies significant economic losses for companies.
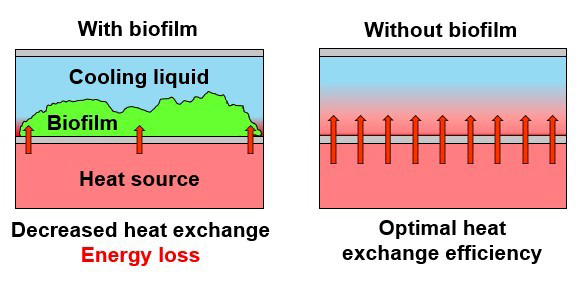
Bio-fouling occurs according to two different mechanisms: accumulation of microorganisms on heat transfer surfaces and attachment of microorganisms on the deposit layer formed on heat transfer surfaces (Liu et al. 2017). Milk fouling and biofilms reduce the effectiveness of milk heat treatment (Yaniarti, Amarantini and Budiarso 2017).
Fouling due to protein and mineral deposits on the surface of plates during pasteurisation also accelerates bacterial adhesion to surfaces, leading to the development of biofilms. The development of biofilms in milk processing environments leads to increased opportunities for microbes to contaminate processed dairy products. In addition, spoilage and pathogenic bacteria trapped in biofilms are protected from disinfectants by multi-species cooperation and the presence of extracellular polymeric substances, which determine their survival (Watnick and Kolter, 2000).
The role of biofilms in post-pasteurisation contamination
Post-pasteurisation contamination is considered a limiting factor in maintaining the quality of dairy products (Phillips et al. 1981; Schroder et al. 1982; Schroder, 1984) and an important commercial factor. Cleaning/sanitising procedures are used to minimise this risk, but a limitation of classical CIP procedures is the adhesion of bacteria to surfaces resulting in the formation of a biofilm (Maxcy, 1964, 1969).
The persistence of adherent organisms in the form of bacterial biofilm can be a source of continuous post-pasteurisation contamination and lead to a reduction in quality and shelf-life (Koutzayiotis, 1992).
For this reason, the adoption of specific protocols to control and prevent biofilm contamination represents an important strategy in reducing the impact of such contamination.
Piramide Environment, thanks to its team of experts, offers the highly specialised ‘Biofilm Expert’ service which includes:
- Analysis of the starting situation
- Development of specific protocols for biofilm removal thanks to the Biorem ® patent
- Implementation of the protocols alongside the client
- Verification of results with state-of-the-art analytical methodologies
- Development of ‘customised’ prevention and monitoring plans
The graph below shows the results obtained in terms of total count reduction following the introduction of Biorem® treatments on a milk pasteuriser.
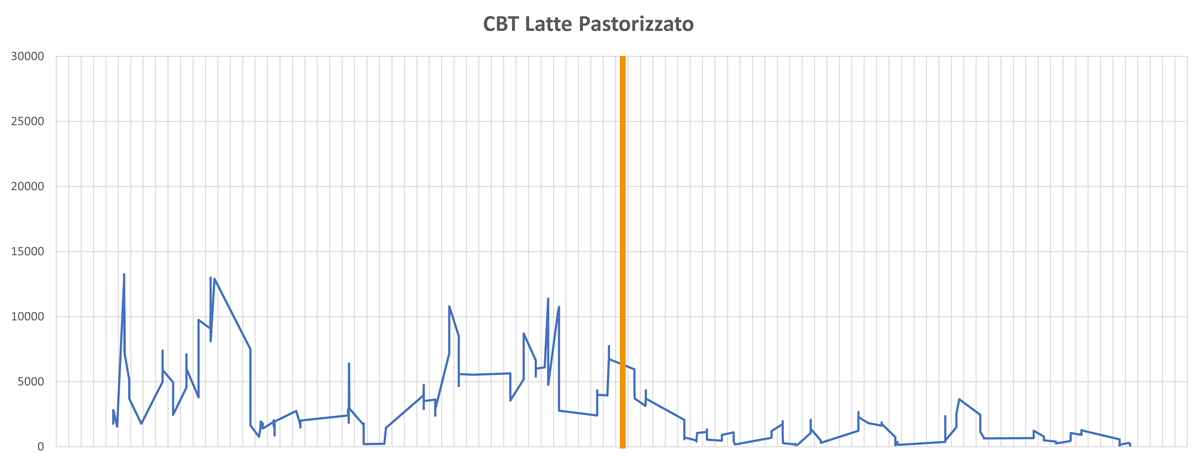
The graph shows the total microbial flora measured in post-pasteurisation milk. The orange line indicates the first Biorem treatment, followed by daily cleaning/sanitisation protocols developed by our staff. It can be seen that after the Biorem treatment and the implementation of the new procedures, the total counts are always below 1000 cfu/mL, whereas before treatment they were often higher.
For more information, ask our experts:
Tel: 0332 826017
Email: av@piramide-ambiente.it



