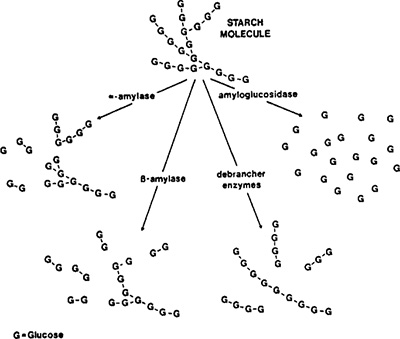
Several yeasts are capable of causing unwanted contamination in beer, and Saccharomyces cerevisiae subsp. diastaticus ( S. diastaticus for short) represents one of the most dangerous contaminating yeasts. Unlike S. cerevisiae, S. diastaticus possesses the extracellular glucoamylase genes STA1-3, which enable it to degrade starch and dextrins.
Glucoamylases are a family of enzymes that remove single glucose molecules from the non-reducing ends of starch and dextrins by hydrolysis of α-1,4 glucosidic bonds (4).
The body of the beer suffers as a result, but not only that: S. diastacus produces an unpleasant phenolic taste, causes turbidity and super-attenuation.
The latter leads to an increased alcohol content and excessive carbonation of the beer.
The latter is the most feared flaw of all.
It is assumed that the costs associated with the incidence of infections due to S. diastaticus range from millions to billions of euros per year in Europe alone. The incidence of S. diastaticus contamination in the brewing industry increased from 1 to 19 cases each year between 2008 and 2016.
Contamination by S. diastaticus occurs in about two thirds of these cases during packaging, most likely originating from the presence of biofilms. It is well known that cells in biofilms have a higher resistance to stress and are not easily removed; the presence of a biofilm represents a high risk factor if one considers that within only 2-13 hours the micro-organisms forming it can re-colonise other locations.
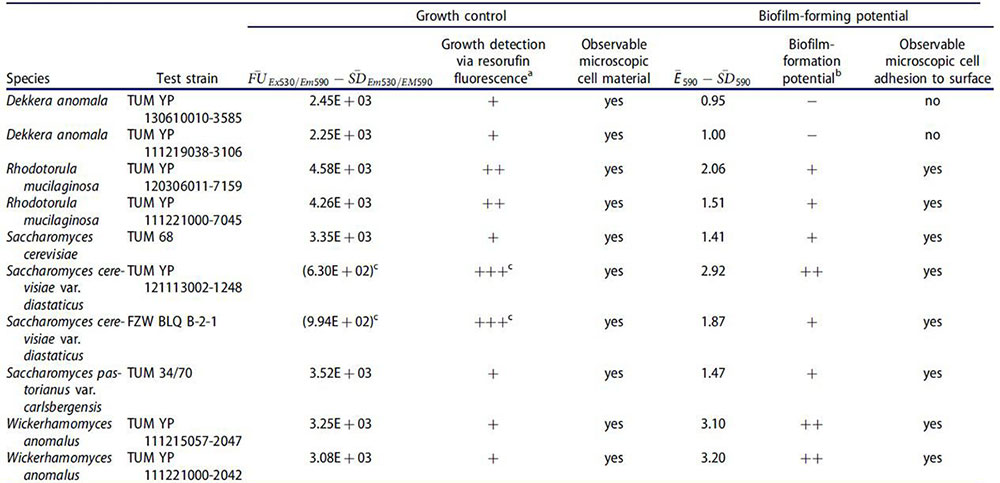
The table shows the growth and potential biofilm formation of selected cultured yeasts, hygienic indicator yeasts and yeasts obligate spoilage yeasts. It is observed that S. diastaticus has a strong biofilmogenic potential.
On the other hand, primary contaminations occur more occasionally, in which the diastaticus yeast competes with the cultured yeast during the fermentation process, especially if diastatic strains are already used in the brewery for production, e.g. of Saison-style beers, or contaminated yeasts are used.
Another source of contamination is the circulation of air in the area of the filling and capping machine. Subsequent pasteurisation of the product is also not always successful in avoiding the harmful effects and consequences of previous contamination due to the ability of S. diastaticus to form ascospores.
Ascospores represent the most resilient phase of S. cerevisiae. For example, they are more resistant to alkaline and acidic conditions than vegetative cells.
However, vegetative cells in stationary phase are equally resistant to frost and desiccation. Furthermore, stationary and quiescent phase cells have similar heat resistance to ascospores up to 42 ◦C, but the latter cells may be more resistant to higher temperatures (above 60°C).
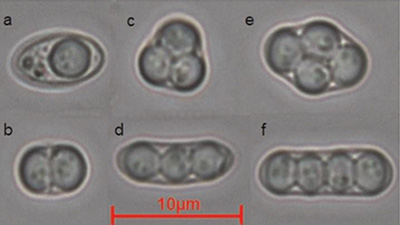
Ascospore resistance can also occur in breweries through exposure to cleaning agents and disinfectants at sub-lethal concentrations.
This is precisely why it is a non-negligible contaminant even in industrial breweries, as the pasteurisation process cannot guarantee any post-contamination on the finished product.
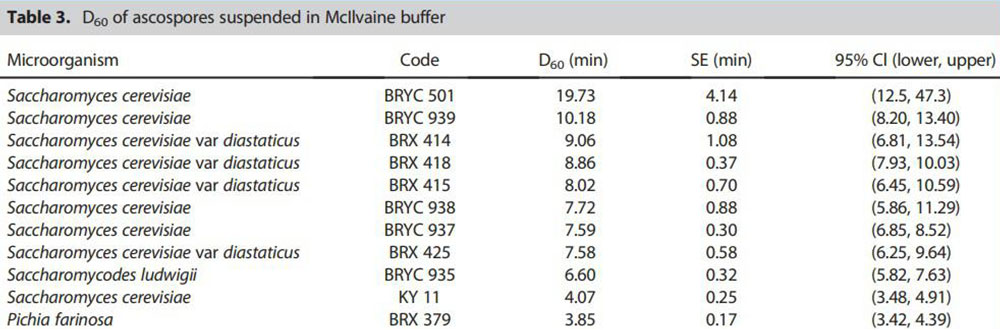
Table – The table shows the heat resistance of ascospores of different Saccharomyces strains. The heat resistance of ascospores of spore-producing yeast strains was determined at one temperature (60°C). The most heat-resistant ascospores were produced by Saccharomyces cerevisiae BRYC 501.
The contaminations found are mostly the result of a random presence, not spread over the entire production, making certain detection on the bottled product very difficult. Depending on the yeast strain S. diastaticus and the corresponding spoilage/damage potential, cell growth rate and the resulting overproduction of CO2, the time required to detect contamination visually or by tasting the product is quite variable.
Studies have shown that storing contaminated product at 2-8°C is not sufficient to ensure a lower risk of S. diastaticus contamination developing; on the contrary, lower temperatures could give S. diastaticus a competitive advantage over other technological strains.
Methods of identification
PCR is a powerful molecular tool that can amplify and detect the presence of specific DNA regions. For diastatic yeast screening, primers are designed to recognise STA genes, which are not present in normal S. cerevisiae strains and can therefore be used for the species-specific identification of S. cerevisiae var. diastaticus (Balogh and Maraz´1996).
Whereas up until a few years ago, PCR technology required significant investment and specialised personnel, today it is possible to incorporate this technology even within the individual brewery thanks to the reduced cost of thermal cyclers and the availability of ready/easy-to-use kits that can be easily used even by less experienced personnel, obtaining reliable results quickly (even within a few hours).

However, although PCR is a very powerful tool, it too has limitations. Indeed, with PCR analysis, it is possible to amplify specific DNA regions for STA genes, but the actual functionality of the proteins cannot be determined. This means that a sample containing dead, non-viable diastatic yeast or yeast containing non-functional versions of STA genes will test positive in a PCR test, without exerting any net effect on the integrity of the sample.
This is why it is always advisable to flank a molecular PCR test with a traditional plate or liquid culture method (combined with Durham’s bell to observe gas formation) to verify the actual diastase capacity of the yeast isolated in your sample.
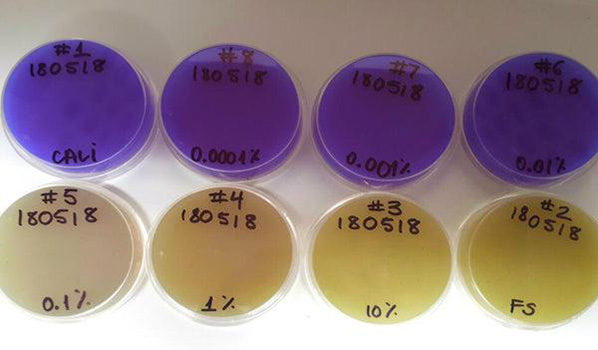

On the left petri plates with selective medium for S. diastaticus. On right durham test with selective broth for S. diastatus
Finally, never overlook certain product features such as:
- Amount of fermentable sugars on finished product close to zero
- Descent of the plato
- Significant increase in alcohol content
- Over-carbonation
Reducing the risk of contamination
If one chooses to use a diastatic strain in the brewery, it is necessary to consider actions to reduce the risk of cross-contamination. The best thing to do first is to designate a specific fermenter dedicated to brewing with diastatic yeast.
Such a tank must always be subject to stringent cleaning/sanitisation procedures, especially if it is used to brew beers with diastatic yeast, or if it is used for different productions. It is always advisable to take the risk of a resistant biofilm into consideration.
In addition to the fermenter, one must also consider:
- Filters/centrifuges
- BBT, Propagators
- Packaging line
References
- Heat resistance acquirement of the spoilage yeast Saccharomyces diastaticus during heat exposure (Inge et al. 2023)
- Presence of Saccharomyces cerevisiae subsp. diastaticus in industry and nature and spoilage capacity of its vegetative cells and ascospores (Inge M. et al. 2021)
- Combined Yeast Biofilm Screening – Characterization and Validation of Yeast Related Biofilms in a Brewing Environment with Combined Cultivation and Specific Real-time PCR Screening of Selected Indicator Species (Riedl et al.)
- Saccharomyces cerevisiae variety diastaticus friend or foe?—spoilage potential and brewing ability of different Saccharomyces cerevisiae variety diastaticus yeast isolates by genetic, phenotypic and physiological characterization (Dornberg et al. 2023)
For more information, ask our experts: Tel: 0332 826017 Email: av@piramide-ambiente.it
Or fill in our FORM



